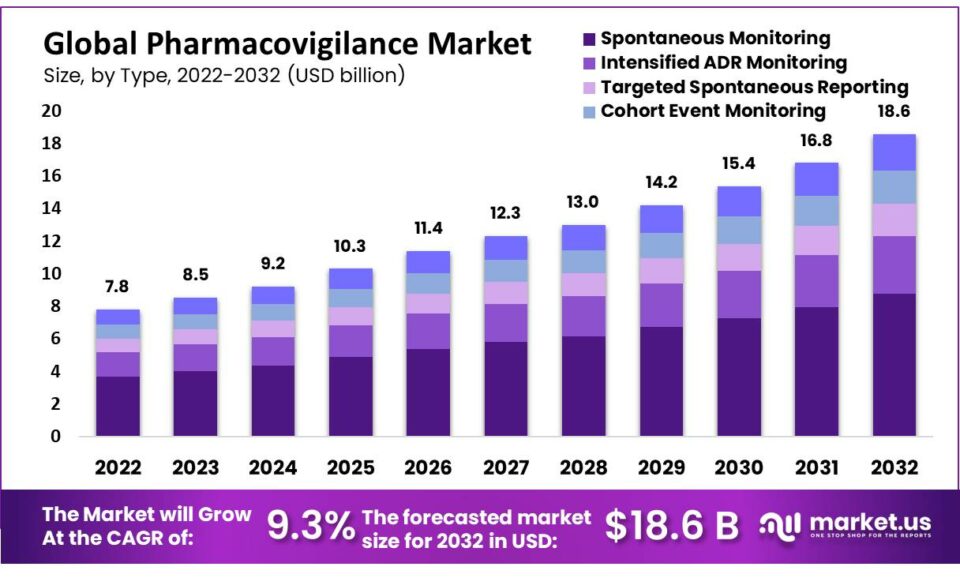
The global pharmacovigilance market size is expected to be worth around USD 19 billion by 2032 from USD 7.8 billion in 2022, growing at a CAGR of 9.3% during the forecast period from 2022 to 2032.
 Get a sample copy of the report to know more https://market.us/report/pharmacovigilance-market/request-sample/
Get a sample copy of the report to know more https://market.us/report/pharmacovigilance-market/request-sample/
Key Market Segments
By Service Provider
- In-house
- Contract Outsourcing
- Others
By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Type
- Spontaneous Monitoring
- Intensified ADR Monitoring
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- Others
By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Pulmonology
- Others
Based By End-User
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Market Key Players
- Accenture plc
- Bristol-Myers Squibb Company
- Clinquest Group B.V.
- Cognizant Technology Solutions Corporation
- GlaxoSmithKline plc
- ICON plc
- Novartis AG
- Hoffmann-La Roche Ltd.
- PAREXEL International Corporation
- Pfizer Inc.
- ICON plc
- Wipro Limited
If You Have Any Questions About This Report, Please Reach Out to Us @ https://market.us/report/pharmacovigilance-market/#inquiry
Drivers
- Regulatory Requirements: Governments enforce stringent drug safety regulations. Compliance with these rules drives demand for pharmacovigilance services.
- Rising Drug Consumption: The global rise in drug consumption, especially for chronic diseases, fuels the need for ADR monitoring.
- Increased R&D Activities: Growing research and development in pharmaceuticals leads to a surge in clinical trials, boosting the demand for pharmacovigilance.
- Emerging Markets: Expanding healthcare infrastructures in emerging markets create new opportunities for pharmacovigilance services.
Trends
- Artificial Intelligence (AI) Integration: AI is revolutionizing pharmacovigilance, improving the efficiency and accuracy of ADR detection.
- Outsourcing Services: Pharmaceutical companies increasingly outsource pharmacovigilance activities to specialized service providers, reducing costs and improving efficiency.
- Electronic Health Records (EHR) Utilization: The integration of EHRs in pharmacovigilance allows for real-time monitoring and more accurate ADR data collection.
- Patient-Centric Approaches: Companies are shifting towards patient-centric pharmacovigilance, involving patients in the ADR reporting process.
Opportunities
- Expansion in Emerging Markets: Expanding pharmacovigilance services in emerging economies presents significant growth opportunities.
- Technological Advancements: Leveraging AI and machine learning can enhance pharmacovigilance processes, offering opportunities for innovation.
- Personalized Medicine: The rise of personalized medicine opens avenues for specialized pharmacovigilance services tailored to individual patients.
- Public Awareness: Increasing public awareness about drug safety can boost the demand for pharmacovigilance services.
Restraints
- High Costs: The high cost of implementing and maintaining pharmacovigilance systems can be a barrier for small companies.
- Complex Regulatory Landscape: Navigating the complex and varying regulatory requirements across different regions is challenging.
- Data Privacy Concerns: The growing focus on patient data privacy may hinder the adoption of pharmacovigilance technologies.
- Skilled Workforce Shortage: A shortage of trained professionals in pharmacovigilance can limit market growth.
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us






